You cannot start recruitment or data collection until you have received written approval from the relevant Human Research Ethics Panel (HREAP) or Human Research Ethics Committee (HREC). Consultations and literature reviews to determine the scope of your project can occur prior to applying for ethics approval.
If your project involves overseas fieldwork, researchers must establish ethics approval before making and confirming travel arrangements.
The UNSW HREAPs and HRECs do not provide retrospective approval for any research conducted without ethics approval or under an expired ethics project.
The HREC and HREA Panels provide approval for up to five years.
The Chief Investigator can apply for an extension before the end date of an approved project. A project modification form must be completed to seek approval for an extension of approval. Please note that:
- Requests for extension can only be granted during the approval period.
- Retrospective extensions will not be granted.
Extensions exceeding 12 months: Extensions for periods of longer than 12 months should be submitted as a new application. The approving HREC/HREAP may grant a 3 month extension to allow the researcher additional time to generate a new application for ethical review.
Extension up to 12 months: A request to extend the approval period for a further 12 months should be submitted as a modification request if recruitment and data collection will cease within a 12 month period. Please note that:
- Additional extensions cannot be requested once an extension has been approved.
- Projects with a high number of modifications submitted during the initial approval period may have to be submitted as a new application.
- Requests for extensions can only be granted if all annual reporting and approval conditions have been met.
External Ethics Approval & Ratification
UNSW has adopted the recommendation of the National Statement to minimise the duplication of ethical review and therefore recognises approvals issued by other NHMRC-registered HRECs and their delegated negligible and low-risk review bodies. This means that UNSW staff and research students do not need to seek ethical review by UNSW HRECs or HREAPs.
Before this type of research can commence, the ethics approval must be registered by the Research Ethics Compliance Support (RECS) Office in the Human Ethics Research Governance process.
Prior to the commencement of the research or participation in an external project UNSW via RECS reserves the right to place conditions on involvement or refuse involvement should approved proposals do not conform to the requirements of the National Statement, other relevant legislation or potentially expose the university to undue risk.
If you have established ethics approval with an NHMRC-registered Australian HREC the submission of a separate application to the UNSW HREC for further approval is not required.
However, UNSW staff and research students are required to register and notify the Research Ethics Compliance Support Human Ethics Team by providing following the external ethics approval process.
Transferring Human Ethics Approval and Moving Institutions
UNSW Human Research Ethics approval only covers the use and storage of data while research personnel are a UNSW staff or student. If you are moving institutions human research ethics approval must be established at the institution before the UNSW HREC or HREAP can approve a transfer request. Information on how to process a transfer request can be found on the following webpage:
https://research.unsw.edu.au/transfer-human-ethics-approval
The review pathway for your human research project is dependent on the level of risk associated with the project. You must select the review pathway specific to the level of risk that your research poses to those that participate in the research. The pathways for review are as follows:
- Pathway 1: Negligible Risk Research – Review by an Executive Panel
- Pathway 2: Low Risk Research – Review by a Human Ethics Advisory Panel
- Pathway 3: More than Low Risk Research – Review by a Human Research Ethics Committee
Following the submission of your human research ethics application, the Human Ethics Office will conduct a compliance check to establish whether the submission requirements have been met. Following the compliance check you will receive an email acknowledging receipt of your submission, you will be provided with a HC reference number and if applicable you will be provided with a list of items that you will need to provide before your submission is added to an agenda for review.
Once all items have been addressed your application will be assigned to a meeting agenda and the application along with the other submissions received will be circulated to the Human Research Advisory Panel (HREAP) or Human Research Ethics Committee (HREC) for ethical review. HREAP and HREC members are provided with the meeting papers at least 7-10 working days before the meeting during this time members will complete their review of each submission. At the meeting the HREAP or HREC members agree upon the points to be addressed and returned to the research team. At the meeting the HREAP or HREC members will agree upon the outcome of the application, there are four possible outcomes from the first stage of formal review approved, subject to, deferred or rejected.
Following the meeting the Human Ethics team will compile the minutes of the meeting and generate the outcome letters for return to the research team. Once approved by the HREAP Convenor or the HREC Chairperson the outcome letters are released via email with all listed members of the research team copied in on the emails. An overview of the ethical review process following a meeting is described below:
- 'Approved' - if your application is approved outright you may commence your research project. You may have conditions of approval to meet*
- ‘Subject To’ - your application has not been approved. You are required to make some revisions to your application and resubmit your updated application, following the resubmission requirements outlined in the outcome notification letter. A decision with subject to requires the applicant to:
- Clarify questions raised by the HREC or HREAP during the review.
- Requests by the HREC or HREAP to revise any items in the application form, project description or the recruitment materials.
- Provide final versions of data collection tools and or recruitment.
Applications that receive a "subject to" decision upon resubmission can reviewed by the Executive which meets weekly:
- HREAP Executive applications close 5pm on a Tuesday for review at the meeting the following Tuesday.
- HREC Executive applications close 5pm on a Tuesday for review at the meeting on Thursday of the same week.
- 'Deferred' - your application has not been approved. You are required to make considerable revisions to your application and resubmit your application, following the resubmission requirements outlined in the outcome notification letter. Your resubmission is reviewed by the full HREAP/HREC at its next monthly meeting. Closing dates for the various HREAP/HRECs are available on the Human Research Closing Dates webpage.
- 'Rejected' - your application has not been approved and elements have been deemed to be unethical. Your research project will not receive ethical approval in its current form. You will be provided with information as to why the research protocol has been deemed unethical. If you intend to proceed with the research, you are required to submit a new application taking into account the reasons outlined in the outcome notification letter.
* Condition of approval: Conditions can be included to allow the research to commence. Examples of conditions include organisational Letters of Support, Therapeutic Goods Administration Clinical Trial Notification, Site Authorisation, Good Clinical Practice Evidence of Training, Evidence of clinical trial registration or a governance related documentation.
Preparing a response for review by the HREAP or HREC Executive
Your application may be approved following the second formal review, or you may be required to make some further changes. If you are required to make revisions or if your application is approved, this will be communicated to you through an outcome notification letter sent via email.
Please respond to the requested information within 1 month from the date of the letter by following the resubmission instructions below. If a response is not received within 1 month from the date of this letter the application will be considered withdrawn and a new application will need to be submitted, unless a request for an extension is received.
- Create a cover letter including the following detail:
- Address your letter to the reviewing HREAP/HREC.
- Include your Project Title and the HC reference number
- Include each point/question raised by the reviewing HREAP/HREC and directly underneath detail the revisions that you have made to address the point or question and confirm that you have made the updates required to the corresponding document. Repeat this step for each point raised.
- Provide copies of the revised Application Form and attachments (as applicable)
- Make any revisions to the appropriate sections of the application form and attachments as requested by the reviewing HREAP/HREC
- Add version numbers or dates to each document.
- Add the HC reference number to all recruitment materials or study tools that will be provided to study participants.
- Provide tracked versions of any amended documents demonstrating the revisions made, followed by a clean version of each document.
- Submission closing date:
- Resubmit your revised application to the reviewing HREAP/HREC for review by the closing date.
- Email an electronic copy to humanethics@unsw.edu.au. The email Subject Heading should read ‘Resubmission of HCXX – Subject To/Deferred etc’
Key Issues in Preparing an Application
Typically, the most important issues to consider within the Application include:
- the supporting rationale for the research
- a detailed and clear description of all procedures/methodologies
- evidence that the researchers have the appropriate qualifications and/or experience and skills to undertake the research
- evidence that demonstrates that the researchers have considered the effects of participation in the research upon the participant(s)
- appropriate procedures to ensure that participants (or legal guardians) provide voluntary and informed consent and can withdraw from the study at any time
- the likelihood and severity of risks and inconveniences posed to participants in this research are identified and the processes in place for minimising these risks and providing support to participants that require it- risks may involve physical, psychological, social and/or legal aspects.
- risks to the researchers or UNSW and/or to other individuals or organisations involved in the research
- appropriate minimisation and management of risks (potential or expected)
- a risk-benefit analysis of the research - where research poses any risks to participants, the researchers, UNSW or other individuals/organisations involved in the research, the researchers need to demonstrate that the benefits of the research outweigh any associated risks
- protection of participants' privacy and confidentiality - related to participant identity and the collection, storage and disposal of data
Literature reviews and meta-analyses do not require ethical approval. While meta data per se do not require ethical approval, there are some instances where you will be required to obtain ethical approval prior to accessing the data set. For example, the data custodian may require you to obtain ethical approval to access the data, or ethical approval may be required as part of the data access licencing agreement.
If you are required to establish ethical approval prior to accessing a publically available dataset then you will be required to follow the data extraction process and licensing requirements of the data custodian in order to gain access to the information. In order to gain ethical approval to access publically available datasets, please complete the UNSW Human Ethics Negligible Risk Application Form.
At UNSW, coursework designed for teaching or learning purposes does not require review or approval via the UNSW ethical review process. It is important to note that coursework projects conducted without human research ethics approval cannot be published for a research purpose at any stage.
Faculties and Schools are responsible for implementing a process for assessing coursework projects to ensure that:
- The activities comply with any relevant privacy and/or confidentiality requirements. (e.g. a process of informed consent);
- Relevant health and safety requirements are adhered to (e.g. blood collection procedures, personal safety procedures, interview protocols etc.);
- Information will not be disseminated or published for a research purpose;
- Participants from a vulnerable population are not the focus of the project (as outlined in section 4 of the National Statement on Ethical Conduct in Human Research, 2015);
- The project does not aim to explore contentious or sensitive topics.
- There is no potential for participants to be exposed to harm as a result of the project including physical, psychological, social, economic or legal harm.
Please contact the human research ethics committees and/or panels for further advice of ethical issues if you have any questions.
Projects involving portraiture at UNSW
At UNSW, portraiture is not deemed to constitute human-research and as such does not require human research ethics approval.
Portraiture is defined here as either:
- The recording and representation of still image, audio and/or moving images recordings of actors or other performers, performing for the purposes of research or course-related work; OR
- The recording and representation of still or moving images of people for the purposes of creative arts practices, where the construction, manipulation and presentation of these portraits images constitutes the primary course-work or research outcome.
Faculty-specific Image Release Agreements (a.k.a. “Model Release Forms”) should be used to provide portrait participants with information about the portrait you are making, the types of situations their image could be shown in, licencing arrangements and limitations on use by (or sale to) third parties, as well as what will happen to this portrait in the event that they no longer wish their portrait to be available for publication/exhibition/circulation. A signed copy of this agreement should be given to portraiture participants for them to keep in the event that they might wish to withdraw their agreement. Faculty-specific Image Release Agreements can be obtained via your supervisor, course-convenor or Head of School.
If you intend to record statements made by the person to be recorded (also referred to as the model / sitter / subject) in which they contribute their personal insights, experiences and/or opinions – this would be considered an interview and would require UNSW human research ethics approval.
If you intend to conduct a survey, an interview or focus group discussion in order to seek feedback about the portrait for a research purpose, this would also be considered human research and therefore ethical approval would be required.
Quality Assurance and Evaluation Activities
The UNSW HREAPs and HRECs do not provide retrospective approval for any research conducted without ethics approval or under an expired ethics project. The guidance on whether or not ethical approval is required should be sought by contacting the Human Ethics Team via email humanethics@unsw.edu.au
Commencement of advertising and recruitment
When research will involve the direct participation of people (e.g. testing, surveys, interviews, focus groups, observation and health or behavioural interventions) the recruitment phase of a project is fundamental to the success of the research. Depending upon the design of a project, recruitment can include such matters as identifying individuals as potential participants, contact between the research team and potential participants, screening or exclusion of some individuals, and preparing to seek consent from the potential participants.
To ensure that your recruitment strategies adhere to the ethical principles of justice and respect you must obtain ethics approval prior to engaging in the recruitment process. Your ethics application should:
- Clearly describe the recruitment strategy and the criteria for the selection of potential participants (National Statement, section 3.1.12);
- Describe and justify the approach to potential participants (i.e. how will potential participants find out about the possibility of participating, or not, in the research) (National Statement, section 3.1.19); and
- Provide reviewers with proposed recruitment materials (e.g. notices, flyers, advertisements, and social media posts) prior to use, including those materials that are developed subsequent to the initial review of the research proposal (National Statement, section 3.1.20).
Stakeholder engagement during the development and design of the recruitment strategy is the only time where prior ethical approval is not required. This would involve engagement with specific groups (generally in person) to discuss the research and, if necessary, to obtain feedback from relevant stakeholders on whether the proposed recruitment strategy is appropriate. It should not involve any of the following:
- identifying individuals as potential participants;
- contact between the research team and potential participants (including advertising for the study);
- screening or exclusion of some individuals; or
- preparing to seek consent from the potential participants.
The purpose of the stakeholder engagement process is to establish whether an organisation may be able to provide access to the intended target population for recruitment purposes. Only after the research proposal has received ethical approval should recruitment commence.
Letters of organisational support confirming their support of the research or their agreement for service provision must be sought by the HREC/HREAP in circumstances where:
- Access to services provided by an organisation for a research purpose. Services for research purposes can include:
- Magnetic resonance imaging, scanning services;
- Specimen collection, pathology services
- Pharmacy and/or dispensing services for research purposes;
- Provision of access to patient and/or corrective service populations and or records held by an organisation;
- Provision of access to organisational policy documentation or procedural information.
- Mailing lists, volunteer registries or personal contact details will be provided to the research team by an organisation where prior consent to share the individuals personal contact details for the purpose of inviting them to take part in research has been obtained.
- Organisations will be asked to introduce the research to anyone involved with their organisation (e.g. stakeholders, clientele, employees, service users, teachers, students) on behalf of the research team. The only exception to the provision of a letter of support in this circumstance where the recruitment strategy specifies that:
- In place of an organisational letter of support before approval the research team will send a recruitment pack with a request to introduce the research by disseminating the recruitment materials. The recruitment pack must include:
- A letter of invitation that provides a short overview of the research with a request to support the study by disseminating an invitation email with a participant information statement and consent form attached or posting recruitment flyer with a link to a downloadable version of the participant information statement and consent form. In order to ensure that recruitment is conducted without the potential coercion or pressure the invitation or flyers must advise those interested that they need to contact the research team to register their interest in participating.
Stakeholder Engagement vs Recruitment
When research will involve the direct participation of people (e.g. testing, surveys, interviews, focus groups, observation and health or behavioural interventions) the recruitment phase of a project is fundamental to the success of the research. Depending upon the design of a project, recruitment can include such matters as identifying individuals as potential participants, contact between the research team and potential participants, screening or exclusion of some individuals, and preparing to seek consent from the potential participants.
To ensure that your recruitment strategies adhere to the ethical principles of justice and respect you must obtain ethics approval prior to engaging in the recruitment process. Your ethics application should:
- Clearly describe the recruitment strategy and the criteria for the selection of potential participants (National Statement, section 3.1.12);
- Describe and justify the approach to potential participants (i.e. how will potential participants find out about the possibility of participating, or not, in the research) (National Statement, section 3.1.19); and
- Provide reviewers with proposed recruitment materials (e.g. notices, flyers, advertisements, and social media posts) prior to use, including those materials that are developed subsequent to the initial review of the research proposal (National Statement, section 3.1.20).
Stakeholder engagement during the development and design of the recruitment strategy is the only time where prior ethical approval is not required. This would involve engagement with specific groups (generally in person) to discuss the research and, if necessary, to obtain feedback from relevant stakeholders on whether the proposed recruitment strategy is appropriate. It should not involve any of the following:
- identifying individuals as potential participants;
- contact between the research team and potential participants (including advertising for the study);
- screening or exclusion of some individuals; or
- preparing to seek consent from the potential participants.
The purpose of the stakeholder engagement process is to establish whether an organisation may be able to provide access to the intended target population for recruitment purposes. Only after the research proposal has received ethical approval should recruitment commence.
Emails to research participants
In order to ensure that the identity and confidentiality of the email recipients is protected, inserting email addresses into the "BCC" field is no longer an acceptable method. As a solution the following methods for emailing research participants are recommended:
Mail Merge Instructions: To create a mail merge using a template email written in Microsoft Word and a list of recipients saved in an excel spreadsheet:
- Start Word. A blank document opens by default. Leave it open. If you close it, the commands in the next step are not available.
- On the Mailings tab, in the Start Mail Merge group, click Start Mail Merge.
- Click Step By Step Mail Merge Wizard. The wizard will guide you through the Mail Merge process and allow you to connect your document to your list of recipients. Emails sent using this process will send to each participant, as opposed to sending to a list of participants which could result in confidentiality breaches. Click here for more information on using mail merge to send e-mails to an address list.
UNSW- Mailman Mailing List Service UNSW IT has a mailing list service which is similar to the above mail merge system. Further instructions on how to access this service can be found by accessing the following link: https://www.it.unsw.edu.au/students/mailman/index.html
Student Participation in Research and Unequal Relationships
In line with section 2.2.9 and 3.1.18(a) of the National Statement researchers must ensure that the outlines the mechanisms that will be put in place to minimise the risk of students experiencing pressure or coercion if they are invited to take part in research being conducted by their course lecturer, convenor or tutor. The presence of an unequal relationship has the potential for students to feel pressured or coerced into participating in research being conducted by their lecturer, convenor or tutor. Students may perceive the act of non-participation as having an impact (or the potential to) on their course grades or exam results and as a result provide their consent to participate. Below are the HREAP/HREC recommended practices for the recruitment of students in research:
Recruitment during lectures, tutorials, presentations or moodle
- Email: A school administrator should be asked to distribute an invitation email with a copy of the participant information statement and consent form attached to students on the research team’s behalf before students attend the course lectures, tutorials or presentations. To remove the potential for students to perceive the act of non-participation as having an impact on their course results the email used to introduce the template must clearly specify that participation in the research is not a course requirement and their decision not to participate will not impact on their course grades. The template wording used in the email invitation or recruitment advertisement examples on the Human Ethics page must be used.
- During Lectures: A study advertisement placed in the lecture theatre near the entry exit that allows students to elect whether to collect one. To remove the potential for pressure or coercion the lecturer course convenor or tutor must not specifically introduce the research project during lectures or presentations. To remove the potential for students to perceive the act of non-participation as having an impact on their course results the email used to introduce the template must clearly specify that participation in the research is not a course requirement and their decision not to participate will not impact on their course grades.
- Moodle: Research study advertisements can be placed in the announcements, news forums and site news sections of moodle and the student recruitment online platform post template provided on the human ethics forms and templates page.
- School Based Participant Pools: Formal student participant pools set up for the purpose of research recruitment exist in some schools. Researchers should enquire with their school administrators to establish what the process is for accessing these recruitment pools.
Informed Consent
- The Participant Information Statement and Consent Form must clarify that participation in the research study is separate from participation in the UNSW Course, and that choosing to/not to participate will not have any impact on students’ grades in the course. If the CI is the lecturer, convenor or tutor of the course, another member of the research team should be listed at Section 10 of the Participant Information Statement & Consent Form for participants to contact for questions about their participation in the research.
- For anonymous surveys, the Participant Information Statement and Consent Form should be attached to the start of the survey so that students can read through the information about the project before completing the survey. Completion of the survey is taken as implied consent.
- For interviews/focus groups/observations/experiments/interventions, the Participant Information Statement and Consent Form should be attached to the recruitment invitation email or should be provided to those who respond to the recruitment poster before data collection. Written consent should be obtained before data collection.
- If you intend to access students’ course grades or Weighted Average Marks (WAMS), informed consent must be obtained from students, and you must explain to participants in the Participant Information Statement and Consent Form the format this data will be accessed in (e.g. non-identifiable/identifiable).
Data Collection
The data collection for the research should not take place during course time. If it is necessary for this occur, the researchers must justify:
- Any course time taken up by the research tasks;
- How the data collection will be completed during course time in such a way that students do not experience coercion or pressure to participate (e.g. who will administer the data collection tool, what options are available for those who choose not to participate);
- How students who do not choose to participate in the study will not be disadvantaged.
Access to Student Data for Research Purposes
- If you intend to access students’ course grades or WAMs, outline who will provide this data to the research team, and in what format (e.g. non-identifiable/identifiable).
Reimbursement and Incentives for Recruitment
Reimbursement for time spent taking part in research activities or of appropriate travel expenses (e.g. parking, taxi or public transport fares) can be offered as incentive for recruitment. Information about reimbursement/incentives along with a justification must be included at section 9 of the project description. Information must also be outlined in any recruitment materials circulated and the process for claiming reimbursement or being notified of prize draw winners must be included in the participant information statement and consent form.
Reimbursement: The provision of gift cards and parking vouchers are generally acceptable forms of reimbursement. If participants are to be provided with a monetary payment for time spent in the research the project description and the participant information statement must specify the maximum amount of money participants will receive or be provided with an hourly rate.
Prize Draws: If you are using prizes, rewards or incentives to recruit research participants, keep in mind that these are considered “games of chance” and are subject to NSW Lotteries Act. It is entirely acceptable to run and promote such competitions, for non-commercial purposes (including as an aid to academic research), provided that:
(a) appropriate ethics approval is obtained,
(b) you don’t offer “cash prizes” – prizes must be limited to non-monetary options, such as gift/store vouchers (not redeemable for cash) or goods,
(c) any advertising is honest and not misleading – and should include some indication of when the competition will be drawn, and
(d) you notify winners promptly (within a day or two) of the prize being drawn. Different types of competitions are subject to different regulations, and can raise additional legal issues. If in doubt, contact the Legal Office on x52701.
Supported Data Storage Platforms
UNSW provides a number of approved data storage systems for our researchers.
- UNSW OneDrive (part of our Microsoft Licence) is suitable for project storage and collaborations. Data stored on UNSW OneDrive will be retained for at least 7 years.
- UNSW Data Archive is suitable for long-term storage of research data. Data stored on the Archive can be retained permanently. It is suitable for use from project start as it protects data files from deletion or changes.
- For a more comprehensive list of storage options please visit our Data Storage and Tools page.
We recommend seeking advice if:
- you need a solution to manage very large datasets
- you need a solution for highly sensitive data (medical, social, cultural etc)
- data storage costs are a significant factor in your grant.
For further advice on data systems or options to store data contact: rdm@unsw.edu.au
Conversion of Paper Records to Digital Records
If the intention is to convert paper records to digital records, the requirements set out in the NSW State Archives & Records (SARA) GA45 must be met. Before converting paper records to digital records, a modification request to seek ethical approval for this change must be submitted to and approved. The requirements of GA45, relevant in these circumstances, specify that:
- Authentic, complete, and accessible scans must be made.
- The scans become the official record and are kept in accordance with all legislative requirements, and;
- The source paper records are kept for quality control purposes for an appropriate length of time after copying (and are then destroyed.) GA45 does not specify a retention period prescribed before destroying the source paper records. UNSW records and archives recommends a minimum of 3 months.
Audio, Photographic and Video Recording Participants
If recordings (video, audio or photographic) of participants are to form part of data collection the research proposal and the participant information statement and consent form must detail how this will occur.
The sections of the application relevant to the research methodology, privacy, data storage, confidentiality and the participant information statement must outline:
- what will be recorded and how (e.g. a digital photo of the participant’s face, an audio/video recording of an interview, or video recording of a teacher delivering a lesson);
- what the alternatives are for participants that choose not be recorded;
- whether the recordings will be transcribed and who will be responsible for transcription;
- whether transcriptions will be verified with the participant and if not why;
- how identifiers will be removed from recordings; (National Statement 3.1.41, b, 3.1.42)
- how the recording will be analysed;
- the period the recording will be retained for; and
- who will have access to the identifiable and non-identifiable versions of the recordings?
- If recordings will be transcribed by a person who is not a listed investigator on the application (such as a professional transcription service or a research assistant) they must be asked to sign aconfidentiality agreement before being provided with access to recordings.
- If a person external to the research team will transcribe recordings the participants must be told within the participant information statement that this will occur, and that the person will be asked to sign a confidentiality agreement. In line with the UNSW procedure for handling research data and material all recordings (video, audio or photographic) are considered source data and must be retained following transcription, unless a justification for its deletion is provided. (National Statement 3.3.10, h)
Focus Group or Classroom Recordings
- If participants decide that they do not want to be video recorded during classroom sessions, they must be provided with an alternative (such as not being in the line of sight of the video camera)
- If participants decide that they do not want to be audio recorded during focus group settings, they must be provided with an alternative (such as the researcher turning off the recording device and not taking written notes during the participants discussion points).
Transferring and Backing up recordings while completing fieldwork
- The research team must outline in the privacy sections of the application how recordings will be stored securely while in the field. The researcher should commit to transferring interviews or focus group recordings immediately after the data collection (or as soon as practicable) onto an appropriate UNSW supported platform.
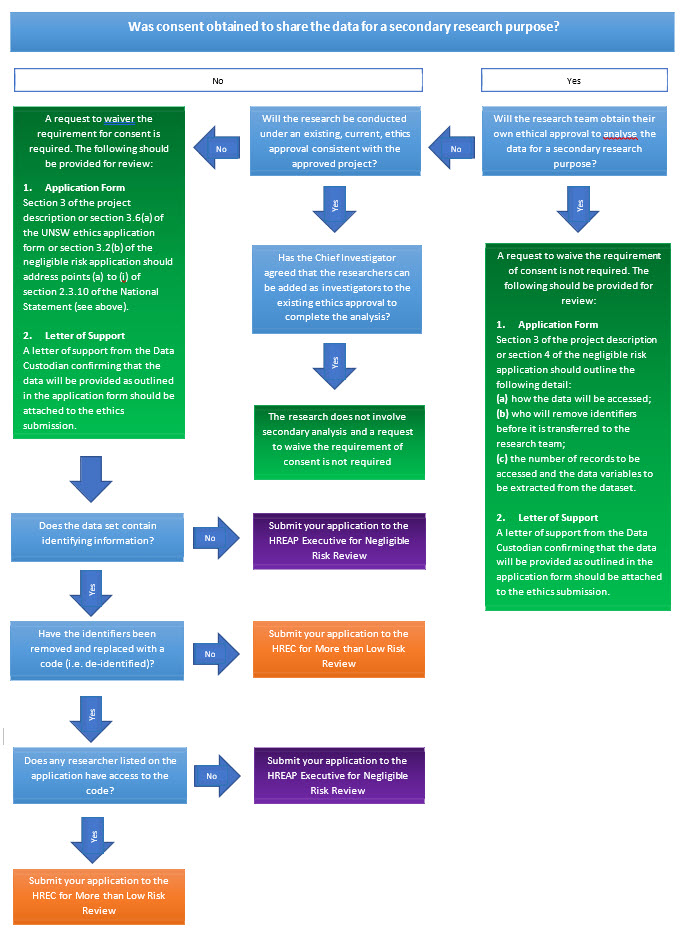
Waiving the Requirement for Consent
What type of consent was obtained by the original researchers:Information collected from an individual should only be accessed and used in a manner that is consistent with the original conditions of consent.
When should a waiver of consent be requested: A waiver of consent (National Statement 2.3.10) maybe requested if the data will be accessed for a secondary research purpose in a manner that is not consistent with the original conditions of consent. The nature of the research or the number of records to be accessed may require an opt‐out approach to consent (National Statement 2.3.5).
Which review body reviews a request to waiver the requirement to obtain participant consent:
- Only an HREC may grant waiver of consent for research using personal information1 in medical research, or personal health information2
-
Only an HREC may grant waiver of consent for research using human biospecimens3 that were collected for clinical purposes.
- A panel or executive may grant waivers of consent for other types of research.
How is a request to waive the requirement for consent sought?
Requests to waive the requirement for consent must be sought by including a justification at section 3.6 (a) of the UNSW Ethics Application Form or section 3.2 (b) of the Negligible Risk Ethics Application Form. The justification must address points (a) to (i) of section 2.3.10 of the National Statement:
(a) involvement in the research carries no more than low risk (discomfort) to participants;
(b) the benefits from the research justify any risks of harm associated with not seeking consent;
(c) it is impracticable to obtain consent (e.g., due to the quantity, age or accessibility of records);
(d) there is no known or likely reason for thinking that participants would not have consented if they had been asked;
(e) there is sufficient protection of their privacy;
(f) there is an adequate plan to protect the confidentiality of data;
(g) in case the results have significance for the participants’ welfare there is, where practicable, a plan for making information arising from the research available to them (for example, via a disease‐specific website or regional news media);
(h) the possibility of commercial exploitation of derivatives of the data or tissue will not deprive the participants of any financial benefits to which they would be entitled;
(i) the waiver is not prohibited by State, federal, or international law.
1 Personal information (i.e. identifiable information) is information or an opinion that identifies a person or could identify them, and includes information about their health. Examples: individual’s name, signature, address, telephone number, date of birth, medical records, bank account details and commentary or opinion about a person (Privacy Act 1988).
2 Health information (i.e. collected for clinical rather than research purposes) is any information about a person’s health or disability, as well as any other personal information collected while they are receiving a health service. It is regarded as one of the most sensitive types of personal information.
3Human biospecimens refers to any biological material obtained from a person including tissue, blood, urine and sputum and any derivative of these, such as cell lines. It does not include non‐human biological material such as microorganisms that live on/in a person.
- Consistent with the requirements of section 4.8.4 of the National Statement, researchers must indicate whether there are ethics approval processes that are relevant to the research in the country in which they intend to do their research, and whether these processes are mandatory or voluntary. Researchers must demonstrate how the research team have investigated this requirement. If the ethical review process is mandatory a copy of the international review boards ethical approval must be provided for noting by the HREC or HREAP before the research can commence in country. See Human Research Establishment of Ethical Approval for more information.
- If there are no relevant ethical review processes in the country where the research will be carried out, as above researchers must demonstrate how they have investigated this requirement. In the absence of a relevant international review board and in line with National Statement 4.8.4, letters of support from a body responsible or involved with the local participant population must support the application and confirm that they were consulted during the design of the research and that there is a plan for continuing consultation with the local participant population. See Human Research Establishment of Ethical Approval for more information.
- In line with the requirements of section 4.8.8 of the National Statement the research proposal must outline the plan (specifically section 10 of the project description) for academic supervision of the student researcher while carrying out the research in country. The plan must outline how the student will be supervised while in the field, what safety measures will be put in place, and the frequency of student supervisor meetings. An appropriate person must be nominated in the country the research will be conducted for the student to seek assistance and guidance from while carrying out the research.
Department of Foreign Affairs and Trade (DFAT) Travel to High Risk Countries
- If research is being conducted in a country where the travel advice is a level 3 – reconsider your need to travel or level 4 – do not travel the research proposal must be reviewed by the human research ethics committee rather than human research advisory panel or its executive.
- The UNSW travel procedure indicates that travel to a high-risk country (travel advice level 3 or level 4) is determined based on the ratings provided by Department of Foreign Affairs and Trade (DFAT) or International SOS (ISOS) and must be first approved by the Director, Risk Management prior to any bookings or travel. In addition, insurance coverage for travel and the research activity must be checked with UNSW insurance before travel. Supporting documentation confirming that this process has occurred must accompany the ethics application before approval is issued.
Participant Information Statement and Consent Form
- For all research whether reviewed by the HREC or a HREAP a local person in country responsible for receiving complaints and concerns in relation to the research project must be included in the Participant Information Statement & Consent Form.
International Ethics Committees (International Review Boards)
- A list of international review boards responsible for providing ethical review of biomedical research can be found by accessing the following links.
- World Health Organisation World-Wide List of International Review Boards
- International Ethics Committee Contact List
Research teams must check before travelling whether a permit to conduct research in country. The following countries have specific laws regarding the establishment of research permits:
-
Indonesia: Govt. Regulation no 41/2006, all foreign researchers intending to conduct research activities in Indonesia must obtain an official permit from the Indonesian authority in advance, including research permit and research visa. A new national science and technology law in Indonesia has recently been passed which imposes penalties on foreign researchers who do not comply with these requirements. Penalties range from blacklisting to fines and possible imprisonment. Whilst this law has been introduced, implementation regulations and guidelines for the law still need to be developed.The attached guide, developed by the Department of Education with the support of the Indonesian Ministry of Research, Technology and Higher Education, outlines the process for applying for the permit and visa. Further information regarding the research permit application process can be found at https://frp.ristekdikti.go.id/.
Supported UNSW Research Data Storage Solutions
UNSW provides researchers with a range of solutions for capturing and storing data then sharing it for reuse.
- OneDrive, part of our UNSW Microsoft Office capability, is suitable for most research projects and is a great way to store and share data during a project.
- eNotebooks are useful tools for capturing data during projects. Pilot implementations of OneNote and LabArchive are currently being run.
- The UNSW Data Archive (dataarchive.unsw.edu.au) is a non-delete system designed to help researchers meet their obligations for long term management of research data. Information detailing where the server is located, who has access and how that access is restricted can be found on the UNSW data archive by accessing the following link.
Further information about research data management, storage and tools can be found on the Research Infrastructure website by accessing this link.
Retention of Research Data and Records
- The university has summarised specific requirements for records retention, including those involving human research data, clinical trials records and ethics records
Conversion of Paper Records to Digital Records
If the intention is to convert paper records to digital records, the requirements set out in the NSW State Archives & Records (SARA) GA45 must be met. Before converting paper records to digital records, a modification request to seek ethical approval for this change must be submitted to and approved. The requirements of GA45, relevant in these circumstances, specify that:
- Authentic, complete, and accessible scans must be made.
- The scans become the official record and are kept in accordance with all legislative requirements, and;
- The source paper records are kept for quality control purposes for an appropriate length of time after copying (and are then destroyed.) GA45 does not specify a retention period prescribed before destroying the source paper records. UNSW records and archives recommends a minimum of 3 months.
Visiting Researchers from Australian Universities
Further information relating to visiting researchers can be found visiting researcher page on the Division of Research website
If recordings (video, audio or photographic) of participants are to form part of data collection the research proposal and the participant information statement and consent form must detail how this will occur.
The sections of the application relevant to the research methodology, privacy, data storage, confidentiality and the participant information statement must outline:
- what will be recorded and how (e.g. a digital photo of the participant’s face, an audio/video recording of an interview, or video recording of a teacher delivering a lesson);
- what the alternatives are for participants that choose not be recorded;
- whether the recordings will be transcribed and who will be responsible for transcription;
- whether transcriptions will be verified with the participant and if not why;
- how identifiers will be removed from recordings; (National Statement 3.1.41, b, 3.1.42)
- how the recording will be analysed;
- the period the recording will be retained for; and
- who will have access to the identifiable and non-identifiable versions of the recordings?
- If recordings will be transcribed by a person who is not a listed investigator on the application (such as a professional transcription service or a research assistant) they must be asked to sign aconfidentiality agreement before being provided with access to recordings.
- If a person external to the research team will transcribe recordings the participants must be told within the participant information statement that this will occur, and that the person will be asked to sign a confidentiality agreement. In line with the UNSW procedure for handling research data and material all recordings (video, audio or photographic) are considered source data and must be retained following transcription, unless a justification for its deletion is provided. (National Statement 3.3.10, h)
Focus Group or Classroom Recordings
- If participants decide that they do not want to be video recorded during classroom sessions, they must be provided with an alternative (such as not being in the line of sight of the video camera)
- If participants decide that they do not want to be audio recorded during focus group settings, they must be provided with an alternative (such as the researcher turning off the recording device and not taking written notes during the participants discussion points).
Transferring and Backing up recordings while completing fieldwork
- The research team must outline in the privacy sections of the application how recordings will be stored securely while in the field. The researcher should commit to transferring interviews or focus group recordings immediately after the data collection (or as soon as practicable) onto an appropriate UNSW supported platform.
Participant Information Statement and Consent Form Information about transcription
- Section 5: If a person external to the research team will transcribe recordings the participants must be told within the participant information statement that this will occur, and that the person will be asked to sign a confidentiality agreement. In line with the UNSW procedure for handling research data and material all recordings (video, audio or photographic) are considered source data and must be retained following transcription, unless a justification for its deletion is provided. (National Statement 3.3.10, h)
- Consent Form: A check box for participants to indicate their permission to be recorded should be included within the consent form.
UNSW will consider conducting an ethical review of research conducted or initiated by an external institution, organisation or company (external entity). An fee of $2500(plus GST) and an annual processing fee applies to all research reviewed on behalf of external entities.
Before proceeding with the review process the following information needs to be provided.
- A brief overview of the aims, participants and outputs of the research and a copy of any measures to be administered to allow the Human Research Ethics Committee or Human Research Advisory Panel that will review the to establish whether it has the relevant expertise required to complete an ethical review of the proposed research.
- An external entities agreement must be established and the fee for review is to be paid.
- Evidence of relevant indemnity insurance.
- A cover letter on the external entities letter head signed off by person with the delegated authority for the External Entity has endorsed the Application for Ethical Review.
The application and research proposal show a commitment to conducting the research in accordance with the principles
Guidelines for Research Involving First Nations Australians
- Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities: Guidelines for researchers and stakeholders.
- Keeping Research on Track II and the Guidelines for Ethical Research in Australian Indigenous Studies (Australian Institute of Aboriginal and Torres Strait Islander Studies 2012).
- Human Research Ethics Committees (HRECs) are also required to apply the Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities: Guidelines for researchers and stakeholders as the basis for assessing proposals for health research with Aboriginal and Torres Strait Islander participation.
Human Research Ethics Committees that specialise in the review of first nations research.
Ethical approval is required for human research involving the collection of blood samples taken from volunteer research participants.
Participant consent must be obtained before blood samples are obtained and the person collecting the blood must be appropriately trained in the technique/s of either finger prick, venepuncture or cannulation. The nature of the training is dependent on the sampling technique being used.
Cannulation and venepuncture can be carried out by any health professional with a current Statement of Attainment from a recognised RTO. Such health professionals would include a Medical Practitioner, Veterinarian, Dentist, Nurse or Exercise Physiologist. If no-one in your School has the appropriate training, you can contact the RECS unit for direction to other qualified people on campus, you could hire a Venepuncturist/Phlebotomist, or you could undertake the relevant training yourself.
The details of the trained health professional responsible for blood collection must be included in the personnel details of the human ethics application form and the safety procedures for collection must be included at section 10 of the project description or attached as a supporting document to the ethics submission.
The Research Ethics Compliance Support Office is currently looking into approved educational institutions to provide a short, competency-based training, which includes recognition of infection control for the injection site, as well as infection protection for the person collecting the blood. You would require a current First Aid certificate in order to receive your Statement of Attainment.
Online Survey or Data Collection Measures
Online platforms are being used more and more to replace paper-based data collection tools. The supported platforms and UNSW for research surveys and data collection are REDCap and Qualtrics. A description of the procedure that will be followed to administer the survey to the participant population. Links to the online survey or data collection tools must be included in the ethics submission for review.
Data Collection Methods
If the data will be collected using an online survey tool or measure the research proposal and recruitment strategy must outline the procedure that the research team will follow in order to administer the online survey to the participant population. Information describing the procedure to administer the survey must be detailed at section 3 of the project description.
Example Wording: The survey or measures will be disseminated by the research team via email during the recruitment process. The survey will be placed on UNSW REDCap platform. The recruitment materials (e.g. email invitation and advertisement) will include a link that provides participants with access to the survey. The Participant Information Statement and Consent Form (PISCF) will be the first page that displays once the link is selected. Only after the PISCF has been read and the participant agrees to provide consent the survey tool or measures will display. The participant completes all survey questions and measures. Following the completion of the survey the research team will extract all data. The survey questions or the measures that the participant will be asked to complete can be accessed by selecting the following link. The link will be included in all recruitment materials.
Recruitment and Consent
The recruitment strategy must outline how the Participant Information Statement will be provided during recruitment and how Consent will be obtained from participants before they progress to the survey questions, this information must be outlined at sections 7 and 8 of the project description.
Example Wording: Study advertisements will be posted on the school webpage and study specific social media pages. The study advertisement will provide a short overview of the research. A link to the survey will be included in study advertisements, those who are interested in taking part will click on the link. The link will display the Participant Information Statement. Once the participants have read through this document, they will be asked to provide consent. Participants can indicate their consent to take part in the research by checking the “I agree, start questionnaire” box.
Survey Questions Recommended Language
The following options are recommended Sexuality and Gender Diversity Indicators
- Sex recorded at birth (ie the sex on your original birth certificate)
- Male
- Female
- Something else (please specify) [open box]
- Prefer not to answer
- How do you describe your gender?
- Man/male
- Woman/female
- Non-binary
- I use a different term [open box]
- Prefer not to answer
- Were you born with a variation of sex characteristics (sometimes called ‘intersex’ or ‘dsd’)?
- Yes
- No
- Don’t know
- Prefer not to answer
- *UNSW is committed to a fair an inclusive workplace free from discrimination, and for this reason it is useful for us to know whether people are heterosexual or non-heterosexual. Would you like to state your sexuality?
- Yes [open text box]
- No
- Prefer not to answer
Alternative sexuality question for instances where the above form is not appropriate:
- How do you describe your sexuality?
- Heterosexual or straight
- Lesbian
- Gay or homosexual
- Bisexual or pansexual
- Queer
- I use a different term [open text box]
- Prefer not to answer
If the research team intends to store the data, information, or biological samples for the initial purpose of their research project only, they will not need to register a databank or biobank unless they seek to archive the data or biological samples beyond the lifecycle of the project in a repository.
If the research team intends to establish a databank or biobank, a data management plan must be developed that addresses their intentions related to the generation, collection, access, use, analysis, disclosure, storage, retention, disposal, sharing and re-use of data and information, the risks associated with these activities and any strategies for minimising those risks (3.1.45, National Statement).
Setting up a research databank requires the researcher to establish the purpose of the Databank and to create operating procedures to define (a) how it will be operated, (b) who will be responsible for the governance of the Databank (c) where the data will be sourced from, (d) how consent will be obtained from donors, and (e) how the data will be stored and released for use in future research. Supporting Documentation required:
- Data management plan
- physical, network, system security and any other technical security measures
- policies and procedures (See Databank Protocol and Databank SOP below)
- contractual and licensing arrangements and confidentiality agreements
- training for members of the project team and others, as appropriate
- the form in which the data or information will be stored
- the purposes for which the data or information will be used or disclosed
- the conditions under which access to the data or information may be granted to others
- what information from the data management plan, if any, needs to be communicated to potential participants?
- Databank protocol
- Name of research databank
- Purpose of research Databank
- Research databank personnel and governing body (i.e. databank review committee)
- Research databank samples or data types
- Collection of data to be deposited into the research databank
- Storage of the data while held in the research databank
- Access to the data held in the bank for further research purposes
- Databank Standard Operating Procedures (SOP)
- Data collection
- Identifying and obtaining participant consent
- Transfer of data to a research databank
- Storage of data within the research databank
- Disposal of data
- Access to data stored within the research databank
- Terms of reference for the governing body reviewing access to data requests
- Establishment of ethics approval
- Data storage log sheets
- Material Data Transfer Agreement Template (for transferring samples from Databank to other researchers)
Researchers should also clarify whether they will seek:
(i) extended or unspecified consent for future research (2.2.14 to 2.2.16, National Statement); or
(j) permission from a review body to waive the requirement for consent (2.3.9 and 2.3.10, National Statement).